Introduction:
Melphalan is a common chemotherapy agent used as conditioning prior to autologous stem cell transplants (Auto SCT). Most frequent side effects are gastrointestinal (GI), with 91% of trial participants reporting symptoms. Among these complaints, diarrhea is the most common with a median onset of symptoms at 7 days and complaints lasting upwards of 17 days. Despite these known post-chemotherapy GI complications, transplant patients are often tested for Clostridium difficile ( C. difficile) given their immunocompromised status. Our study evaluated the rate of C. difficile testing in post-transplant patients to explore its utility and possible overuse in this population.
Methods:
During 2017-2022, patients who underwent Melphalan conditioning prior to Auto SCT were reviewed for C. difficile testing during transplant admission. We analyzed the timing and the results of C. difficile tests obtained during admission. All C. difficile tests obtained prior to admission or following discharge were excluded. From 2017-2020, a positive C. difficile test at our institution required only a positive C. difficile PCR test. Criteria for a positive C. difficile test changed beginning 2021, requiring both a positive C. difficle PCR and toxin antigen tests.
Results:
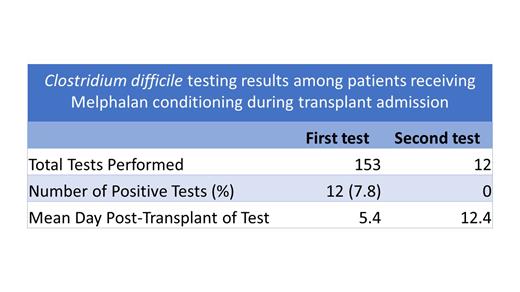
Of the 202 patients included in our database, 153 were tested for C. difficile during their transplant admission. 12/153 tests were positive (7.8%) with the average day of testing being 5.5 days post-transplant. Twelve patients were tested a second time during the same admission with the second test occurring on an average on day 12 post-transplant. All repeat tests were negative for C. difficile. All C. difficile tests following the implementation of the toxin assay resulted negative. Prior to the toxin assay, all positive PCR tests were treated with oral vancomycin.
Conclusion:
Our institutional data indicates that excessive C. difficile testing may occur in the setting of Melphalan conditioning. C. difficile testing was noted to be of low yield with positive rates in only 7.8% of patients. Furthermore, the positivity rate dropped to 0% among our cohort after implementation of the toxin assay in 2021. The mean cost of a single C. difficile test at our institution over 2017-2022 was $29.00 and this excessive testing leads to unnecessary financial burden incurred during the admission. Diarrhea occurring post-auto SCT in this population may be better explained as a side effect of Melphalan conditioning. Our data suggest it may be reasonable to withhold or delay testing until at or after day 7 post-transplant. In addition, repeat C. difficile testing within the same admission may be futile with all cases in our cohort resulting negative, further supporting our conclusion that excessive testing occurs in this population. We will further study our data as part of a targeted quality-improvement initiative to assess post-transplant outcomes with immediate versus deferred C. difficile testing.
Disclosures
Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Takeda: Consultancy, Honoraria; Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Kota:Incyte: Research Funding; Pfizer: Honoraria; Kite: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal